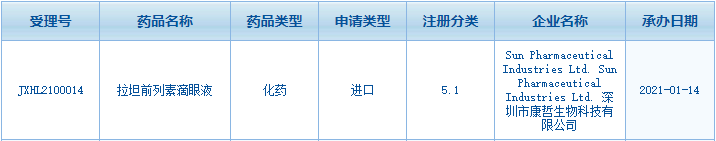
On January 14, the
IND application of CMS’s innovative drug Latanoprost Eye Drops was
accepted by the Center for Drug Evaluation, NMPA. Latanoprost Eye Drops is
a once-a-day, BAK-free novel 0.005% latanoprost ophthalmic formulation, which
is indicated for reduction of elevated intraocular pressure in patients with
open-angle glaucoma, or ocular hypertension.
Latanoprost Eye Drops has been approved for
marketing by the U.S. FDA under the brand name XelprosTM. As a prostaglandin
drug, which is one of the first-line treatments for open-angle glaucoma or
ocular hypertension, the product is developed with LipixelleTM technology that dissolves latanoprost with non-ionic surfactants, and can achieve
BAK-free delivery to avoid the potential ocular damage caused by long-term use
of BAK.
The prevalence of primary open-angle
glaucoma with ocular hypertension in China is estimated to be 1.5% to 2%, with
more than 20 million people. We look forward to the launch of Latanoprost Eye
Drops in China, so as to bring a safe and effective treatment option for
patients with related indications. In the future, as a candidate of the Group’s
innovative drugs in the ophthalmology line, the product will create synergy
effects with other ophthalmic drugs, improving the competitive advantage of the
Group in the ophthalmology line and injecting impetus for the continuous development
of Group’s ophthalmology business.
