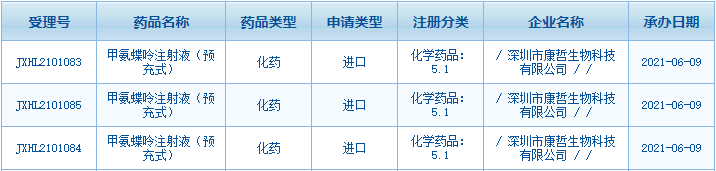
On June 9, the IND application of China Medical
System’s innovative drug Methotrexate Pre-filled Injection was accepted by the
Center for Drug Evaluation, NMPA. Methotrexate Pre-filled Injection is
available in multiple low dose formulations in a small volume and indicated for
the treatment of active rheumatoid arthritis (RA) and other autoimmune diseases
in adults.
Methotrexate (MTX) is internationally well
accepted as the first-line gold standard medicine and anchored agent for the
systemic treatment for RA. While oral MTX has poor patient compliance due to
its relatively severe gastrointestinal side effects, Methotrexate Pre-filled Injection
is administered subcutaneously, and shows better bioavailability, significant improvement
of clinical efficacious response, and convenience of dosage management,
achieving a greater balance between efficacy, safety, tolerability and
compliance. The product has been approved for marketing in Europe.
RA is one of the major autoimmune diseases.
As stated by the “Chinese Rheumatoid Arthritis Diagnosis and Treatment
Guidelines 2018”, the incidence of RA in Mainland China is 0.42%, with around 5
million patients in China. Currently, no MTX injection (including pre-filled products)
for the treatment of RA has been marketed in China. Methotrexate Pre-filled Injection
is expected to be the first MTX injection for subcutaneous administration for
the treatment of RA in China, meeting the medical needs of basic treatment for RA
patients.
